

BSP Pharmaceuticals has capabilities to manufacture conventional and special oral solid formulations on development an, clinical and commercial scale:

AMONG ITS ORAL SOLID TECHNOLOGIES,
BSP OFFERS
Secondary packaging for oral solid forms includes blisters with components such as ALU-ALU; PVC/PVDC and bottling both for tablets and capsules.
A team dedicated to oral solid dosage forms supports the projects at all stages with suitable equipment and analytical instruments specifically designed for the scale up of development processes to the GMP area.

IT IS A SPECIAL CAPABILITY FOR HIGH POTENT ANTICANCER
PRODUCTS WITH LOW SOLUBILITY AND LOW BIOAVAILABILITY.
CAPSULES CAN BE FILLED WITH DISPERSION OR SOLUTION OF
THE API IN AN OILY BASED FORMULATION.
EQUIPMENT
ORAL DEPARTMENT
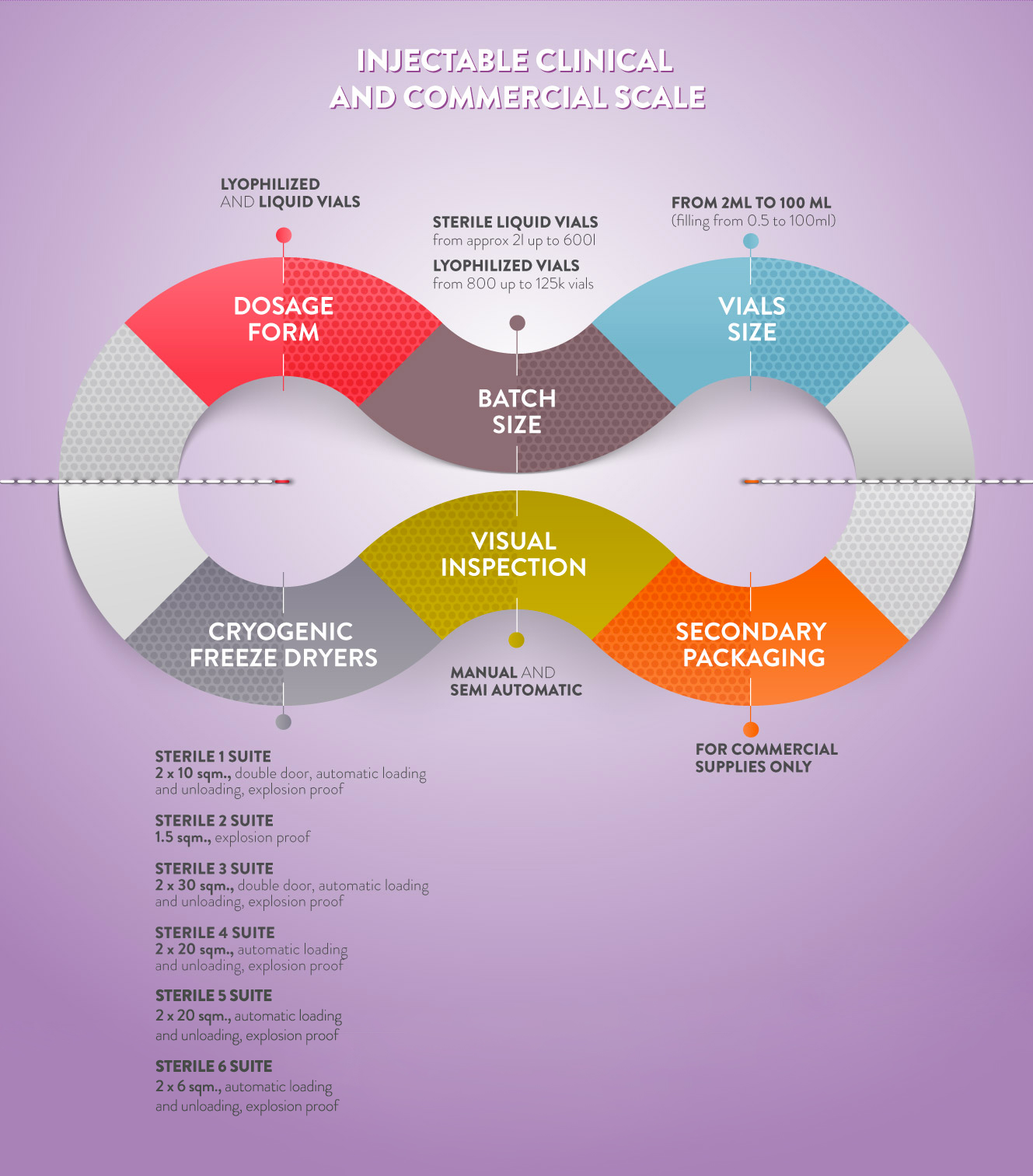
STERILE DEPARTMENTS ARE EQUIPPED TO MANUFACTURE STERILE LIQUIDS AND LYO PRODUCTS.
Primary packaging for sterile dosage forms covers vial dimensions from 2 ml up to 100 ml.
All sterile departments have a system for filling liquid and lyophilized products, as well as oxygen-sensitive products.
BSP can handle liquid or lyophilized lipid-based formulations, such as emulsion and suspension, by using special technologies and equipment, including:
THE STERILE 1 PRODUCTION AREA IS DEDICATED TO THE MANUFACTURING OF LIQUID
AND LYOPHILIZED VIALS FOR CLINICAL AND COMMERCIAL BATCHES.
The FILLING LINE is fully automated and allows the manufacturing of batch sizes from 20L to a maximum of 250L.
VIAL SIZES range from 2ml to 100ml.
The FILLING OPERATIONS are performed by a combined dosage system equipped with volumetric rotary piston pumps and peristaltic pumps, with four filling needles, automatic non disruptive in-line weight check system.
THE LINE is installed under cRABS to assure high containment.
MANUFACTURING OF LYOPHILIZED PRODUCTS is carried out by two cryogenic freeze driers of 10sqm each, explosion proof, with automatic loading and unloading system (ALUS System).
THE LINE is equipped with two external vial washing machines.
THE STERILE 2 PRODUCTION AREA IS DEDICATED TO THE MANUFACTURING OF LIQUID AND LYOPHILIZED VIALS FOR small scale CLINICAL AND COMMERCIAL BATCHES.
The FILLING LINE is automatic low speed and allows the manufacturing of batch sizes from 2L to a
maximum of 100L.
VIAL SIZES range from 2ml to 100ml.
The FILLING OPERATIONS are performed by a peristaltic dosing system.
THE LINE is installed under isolator (sterilized in-situ by using VHP) to assure a high containment and the protection of the operators and the product.
MANUFACTURING OF LYOPHILIZED products is carried out by cryogenic freeze drier 1.5 sqm, explosion proof.
THE LINE is equipped with an external vial washing machine.
THE STERILE 3 PRODUCTION AREA IS DEDICATED TO THE MANUFACTURING OF LIQUID AND LYOPHILIZED VIALS FOR COMMERCIAL BATCHES.
The FILLING LINE is fully automated and allows the manufacturing of batch sizes from 40L to a maximum of 650L.
VIAL SIZES range from 2ml to 100ml.
The FILLING OPERATIONS are performed by a combined dosage system equipped with volumetric rotary piston pumps and peristaltic pumps, with eight filling needles, automatic non disruptive in-line weight check.
THE LINE is installed under cRABS to assure high containment.
MANUFACTURING OF LYOPHILIZED products is carried out by two cryogenic freeze driers of 30 sqm each, explosion proof, with automatic loading and unloading system (ALUS System).
THE LINE is equipped with two external vial washing machines.
THE STERILE 4 PRODUCTION AREA WILL BE DEDICATED TO THE MANUFACTURING OF LIQUID AND LYOPHILIZED VIALS FOR COMMERCIAL BATCHES.
The FILLING LINE is fully automated and allows the manufacturing of batch sizes from 20L to a maximum of 400L.
VIAL SIZES range from 2ml to 100ml.
The FILLING OPERATIONS are performed by a combined dosage system equipped with volumetric rotary piston pumps and peristaltic pumps, with six filling needles, 100% automatic non disruptive in-line weight check.
THE LINE is installed under cRABS to assure high containment.
MANUFACTURING OF LYOPHILIZED PRODUCTS is carried out by two cryogenic freeze driers of 20sqm each, explosion proof, with automatic loading and unloading system (ALUS System).
THE LINE is equipped with external vials washing machine.
THE STERILE 5 PRODUCTION AREA WILL BE DEDICATED TO THE MANUFACTURING OF LIQUID AND LYOPHILIZED VIALS FOR COMMERCIAL BATCHES.
The FILLING LINE is fully automated and allows the manufacturing of batch sizes from 20L to a maximum of 400L.
VIAL SIZES range from 2ml to 100ml.
The FILLING OPERATIONS are performed by a combined dosage system equipped with volumetric rotary piston pumps and peristaltic pumps, with five filling needles, 100% automatic non disruptive in-line weight check.
THE LINE is installed under cRABS to assure high containment.
MANUFACTURING OF LYOPHILIZED PRODUCTS is carried out by two cryogenic freeze driers of 20sqm each, explosion proof, with automatic loading and unloading system (ALUS System).
THE LINE is equipped with external vials washing machine.
THE STERILE 6 PRODUCTION AREA WILL BE DEDICATED TO THE MANUFACTURING OF LIQUID AND LYOPHILIZED VIALS FOR COMMERCIAL BATCHES.
The FILLING LINE is fully automated and allows the manufacturing of batch sizes from 20L to a maximum of 250L.
VIAL SIZES range from 2ml to 100ml.
The FILLING OPERATIONS are performed by a combined dosage system equipped with volumetric rotary piston pumps and peristaltic pumps, with two filling needles, 100% automatic non disruptive in-line weight check.
THE LINE is installed under cRABS to assure high containment.
MANUFACTURING OF LYOPHILIZED PRODUCTS is carried out by two cryogenic freeze driers of 6 sqm each, explosion proof, with automatic loading and unloading (ALUS System).
THE LINE is equipped with external vials washing machine.
BSP PHARMACEUTICALS IS SPECIALIZED TO SUPPORT THE MANUFACTURING OF ADC DRUG PRODUCTS FROM THE CONJUGATION TO THE FILL FINISH.
Manufacturing lines, process flows as well as storage capabilities have been specially designed taking into consideration the critical process parameters and the risk factors associated with the handling and production of this class of compounds.
DEVELOPMENT & GMP MANUFACTURING
ANALYTICAL SERVICES
Dedicated capabilities are installed to support ADC characterization and manufacturing activities (IPCs as well as full release of the product). A specific area of the QC lab has been fully assigned to cytotoxicity studies to carry out testing on determination of ADCs potency.